I can’t take credit for this blog post.
It was written by Tucker Watson, our AmeriCorps Patrol Member who runs our Swim Guide Lab.
Dive in and learn about pH and D.O.!

Many of you rely on OUR Swim Guide program each summer to learn if it is safe to swim based on our weekly bacteriological (E. coli) results. What you might not know about are the various other water quality data we record! In addition to bacteria, there are many other indicators that inform us about water quality conditions for families, fish, and wildlife who rely on the river.
Did you know only 11% of Alabama’s waterways are assessed? The data we collect is not just to educate the public about water quality conditions each week– we also want to monitor long term water quality conditions on the Coosa.
Take a few minutes to learn about two of the other water quality parameters that we collect weekly during the Swim Guide program that are extremely important to the health of the creeks and lakes so you can be even more informed about the health of the rivers.
pH: what is means and why it matters
Definition: pH is simply a measurement of how acidic or basic something is. For example, vinegar is acidic and has a pH of about 3 while bleach is basic and has a pH of about 13. The figure below illustrates various examples of the pH ranges of different organisms and solutions. 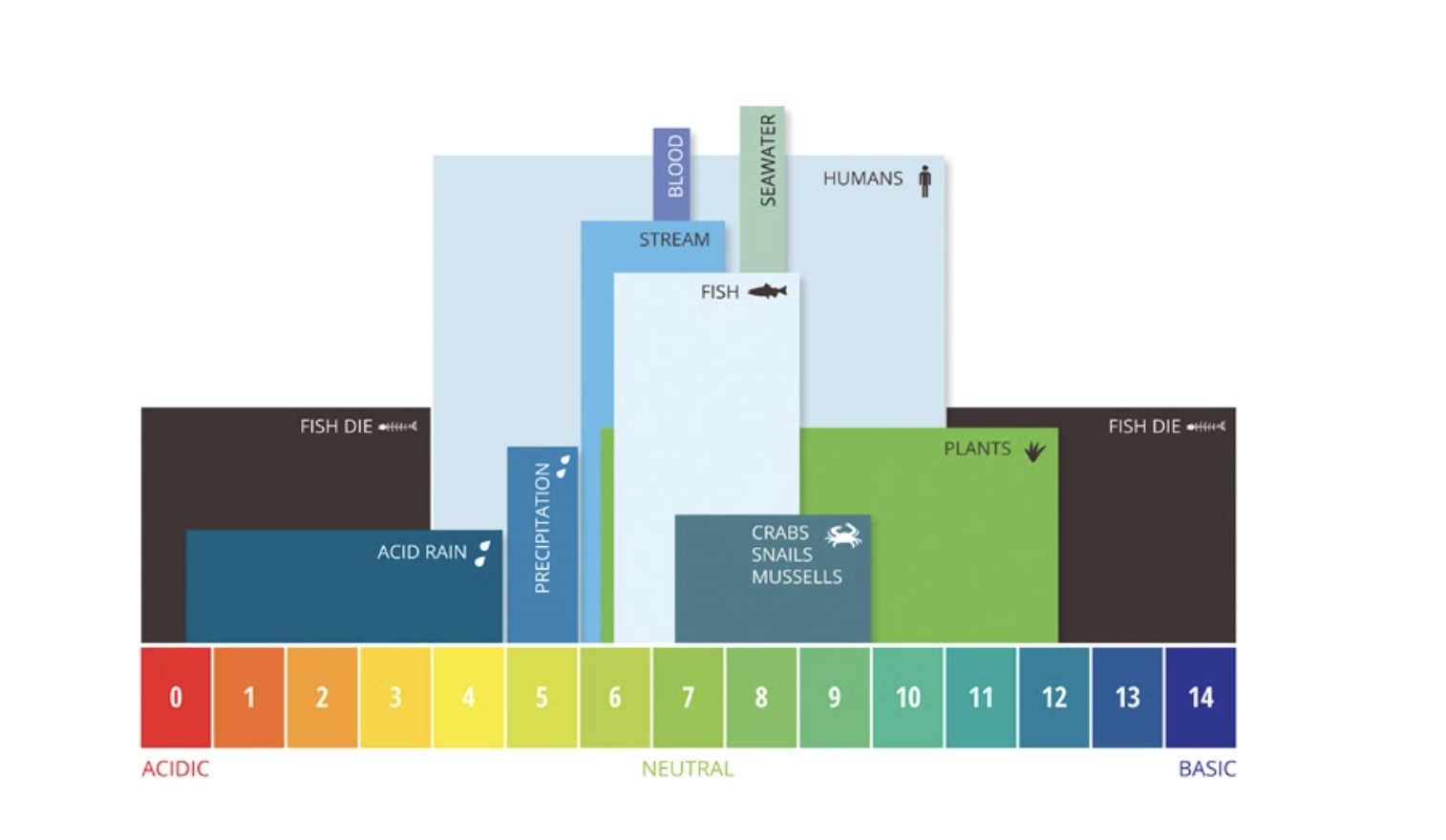
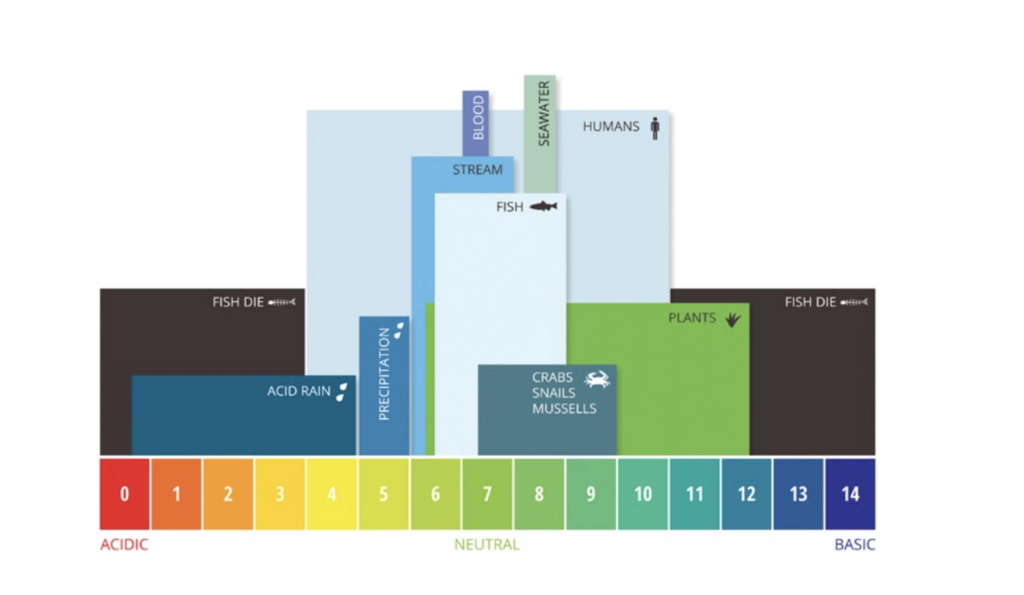
pH is one of the most important factors for healthy water quality. Freshwater ecosystems like the Coosa River can tolerate a narrow pH range (6.0-9.0) before the health of the river, fish, and mussels, start to suffer. High or low pH can indicate pollution or discharge of a chemical or substance that can negatively impact aquatic health.
Dissolved Oxygen, how fish breathe
Definition: Just as humans rely on clean oxygen to breath, aquatic creatures such as fish, mussels, and aquatic microorganisms rely on the amount of dissolved oxygen in the water to breath.
Freshwater ecosystems prosper when dissolved oxygen levels are above 6 (ppm or mg/L) as shown in the figure below. If the dissolved oxygen drops below 3 (ppm or mg/L) in an aquatic ecosystem; fish, mussels, and other aquatic organisms are susceptible to large scale population kills.
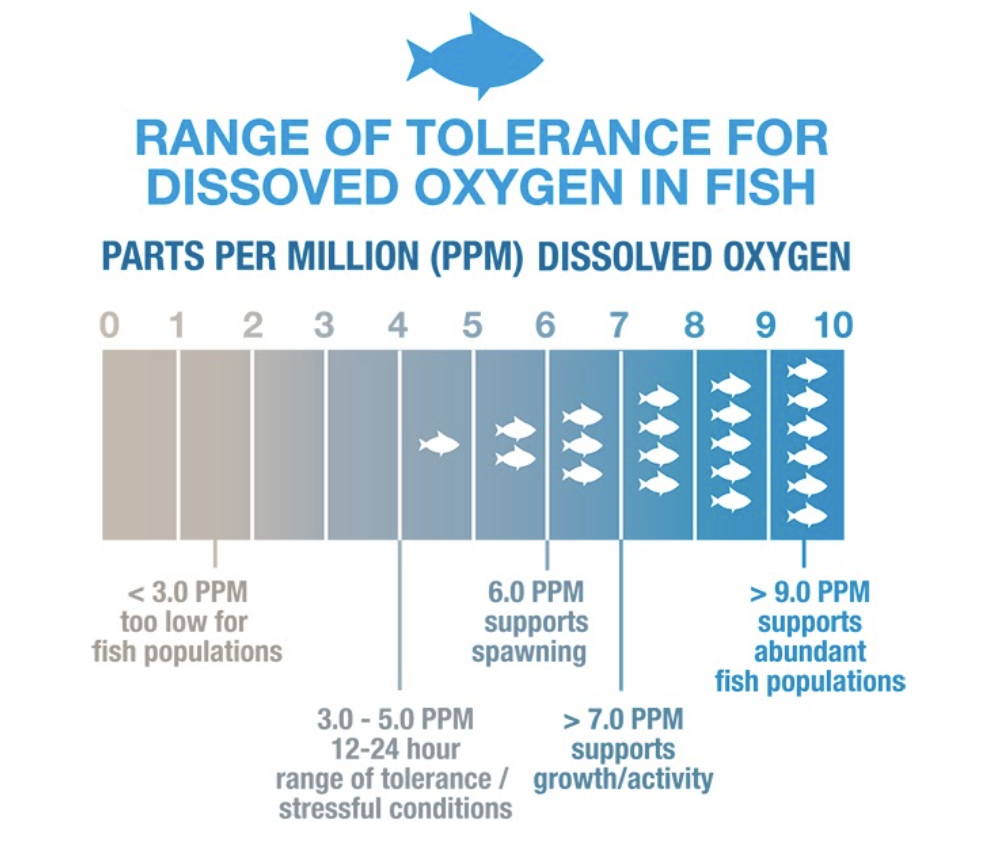
Many different factors can affect dissolved oxygen (DO) levels which is why constant monitoring is important, especially during the summer months. Low dissolved oxygen typically can indicate that some collection of organisms are consuming the majority of the dissolved oxygen in an area. Common organisms that deplete oxygen in freshwater ecosystems include microorganisms (E. coli) and algal populations.
Since aquatic organisms such as fish and mussels rely on dissolved oxygen to live, DO can be an important factor to consider for fisherman. During the warmer summer months, aquatic organisms thrive and consume large amounts of dissolved oxygen. Most freshwater fish can live and reproduce in areas with a dissolved oxygen content of 6 mg/L (or ppm) or greater.



